Introducing Herasight's IVF Calculator
Full Distributions at Each Stage of IVF
The Herasight team is excited to announce our new calculator for predicting IVF outcomes, available at herasight.com.
IVF represents a $25 billion global industry serving millions of couples annually1. Despite this scale, most patients rely on prediction tools that provide only point estimates or a single success percentage, without ranges of uncertainty or the ability to update as treatment progresses. Unlike existing tools, our calculator provides detailed insights into the full range of possible outcomes at every stage of IVF. Additionally, patients can input their known results at any stage of the IVF process to get more accurate and personalized estimates.
The limitations of current tools have clinical consequences. Studies show that patients often discontinue treatment due to the psychological burden of unclear expectations during the IVF process rather than poor medical prognosis2. Without understanding how retrieval outcomes affect downstream probabilities, patients and clinicians lack data for informed decision-making.
Our calculator, built on 539,215 observations from real IVF cycles and clinical studies, addresses these limitations through a fundamentally different approach.
Table of Contents
The Problem with Existing Calculators
When patients use existing IVF prediction tools, they encounter frustrating limitations. For example, the CDC IVF Success Estimator and OPIS calculators only predict your final probability of achieving a live birth. Neither tool can predict how many eggs you will retrieve, how many might fertilize, or how many embryos will be chromosomally normal. These intermediate outcomes matter enormously for treatment decisions.
Orchid’s Embryo Banking Calculator does break down the IVF process by stage, but only provides point estimates of "17 eggs retrieved" or "5 euploid embryos", without a range of possible outcomes. A prediction of “5 euploid embryos” means something different if the actual range is 4–6 versus 1–10.
Additionally, existing calculators cannot update predictions when outcomes become known during treatment. If you retrieve 10 eggs instead of the predicted 17, current tools are not able to take this information into account. Patients are left with the original estimates that no longer match their reality, making it impossible to get accurate guidance.
These limitations matter because 92% of patients prefer shared decision-making with physicians3, and many would benefit from tools that present probabilistic information in more accessible formats4. When current tools hide uncertainty and cannot respond to actual outcomes, patients and doctors lack the information needed for personalized treatment planning.
Our Technical Approach
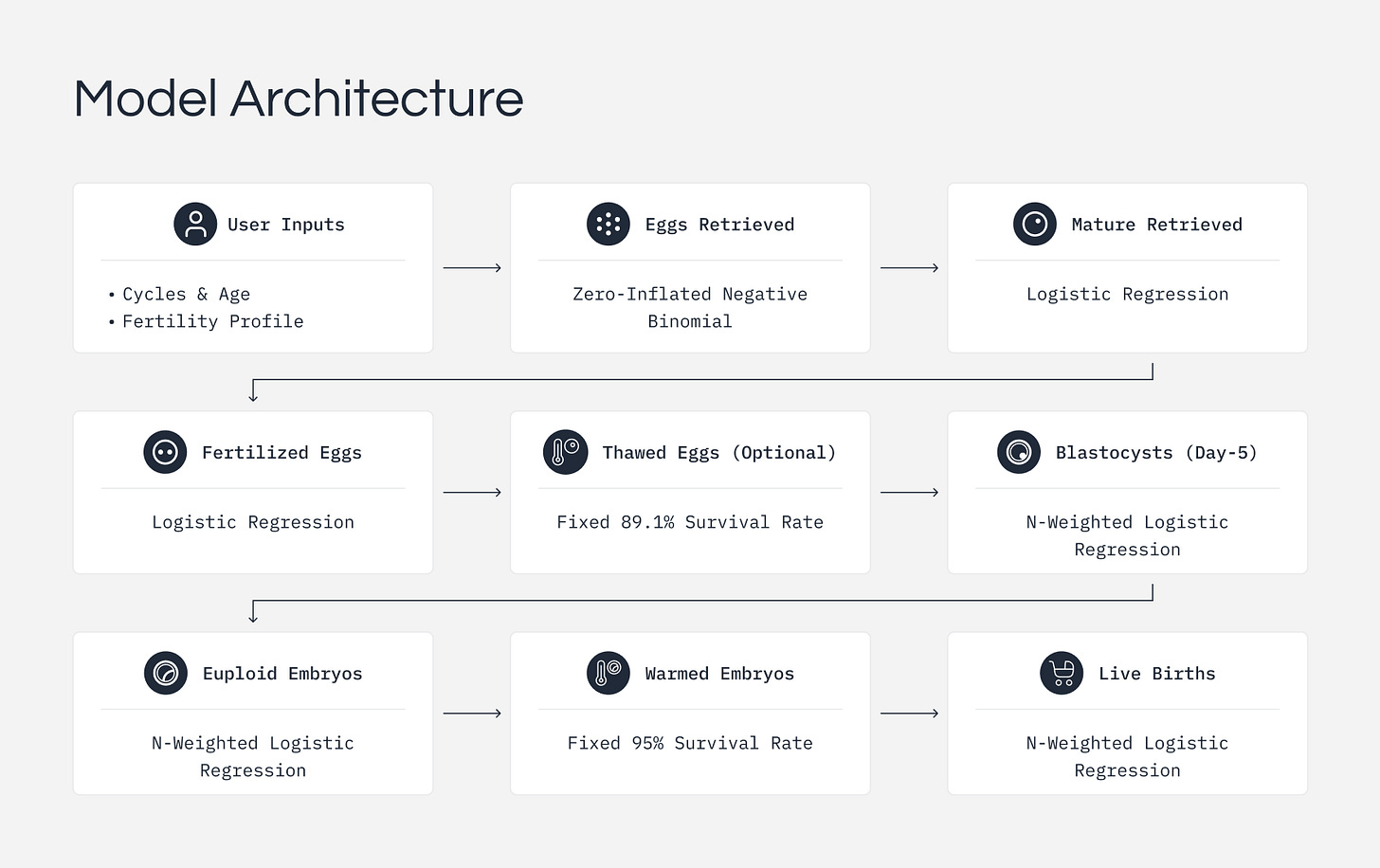
We modeled IVF as a series of biological stages: retrieval, maturation, fertilization, blastocyst development, genetic testing, and implantation. At each stage, some eggs or embryos do not make it through. Our calculator models this filtering process and introduces three key advances
Full probability distributions
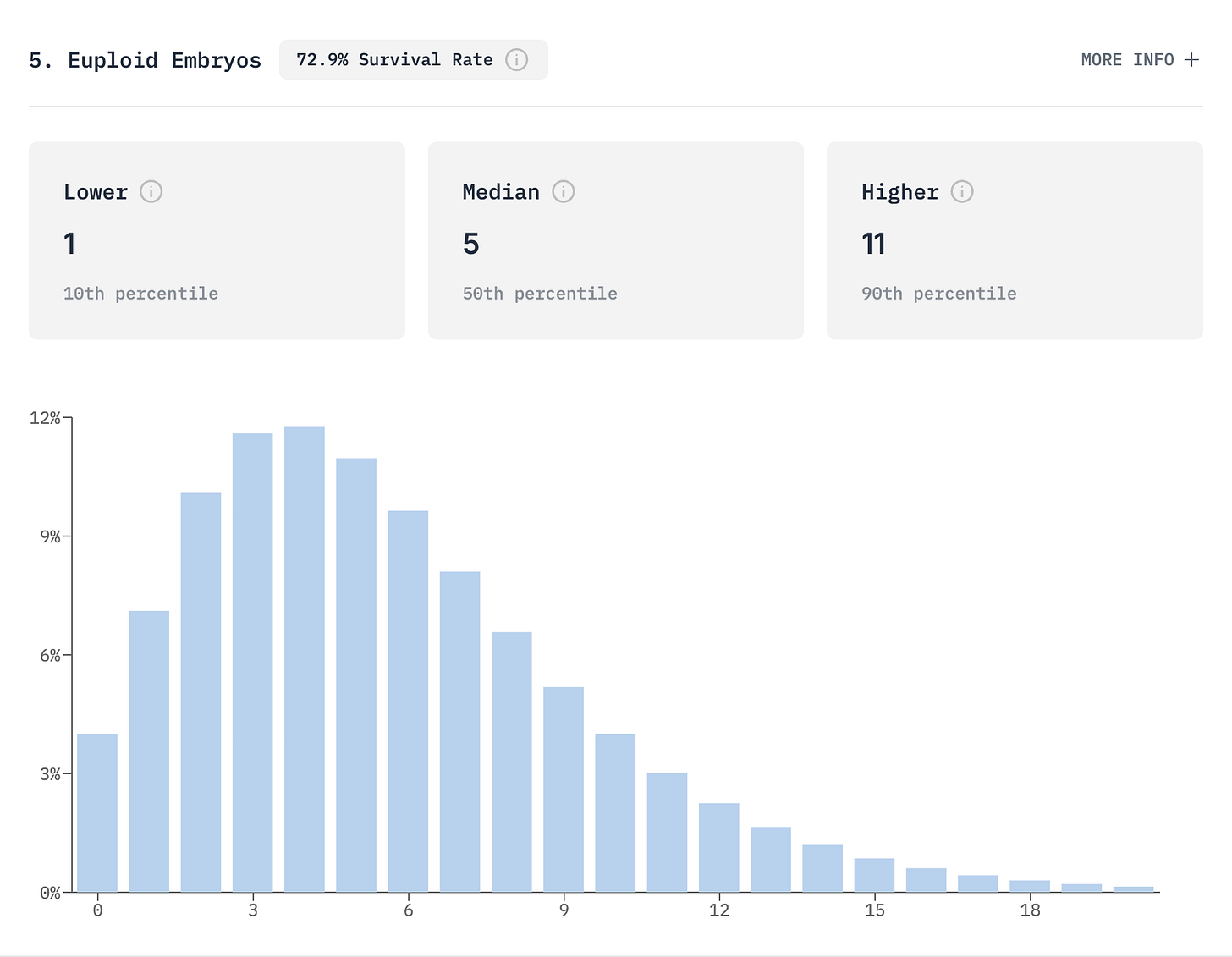
Instead of predicting “5 embryos,” we show the complete distribution of possible outcomes. You might expect 5 embryos, but the actual outcome could range from 1 to 11. This distribution matters for setting realistic expectations and making informed decisions.
Real-time updating
As your IVF cycle progresses and actual results come in, such as retrieving 25 eggs instead of the predicted 17, our model updates to reflect your current situation. The known outcome at a given stage becomes a fixed starting point, and all downstream predictions are recalculated.
Multi-cycle planning
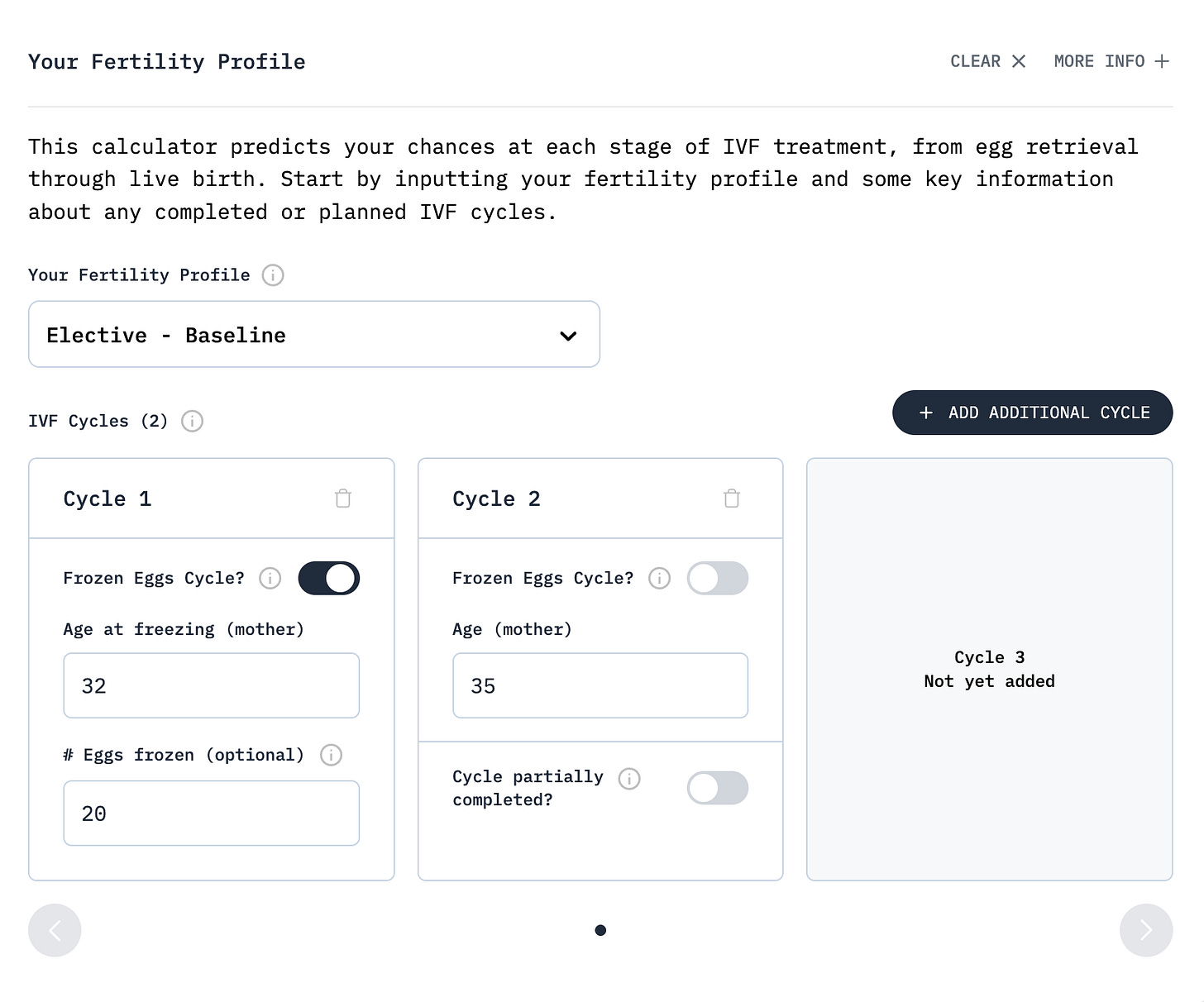
A key practical issue other tools overlook is that many patients combine eggs retrieved at different ages. Our calculator uses discrete convolution to combine these cycles, producing a distribution that captures the true probability spread. Crucially, each age cohort maintains its own success rates: eggs frozen at 32 keep their 76% euploidy rate even when combined with eggs retrieved at 35 with a 71% euploidy rate.
Dynamic Updating in Practice
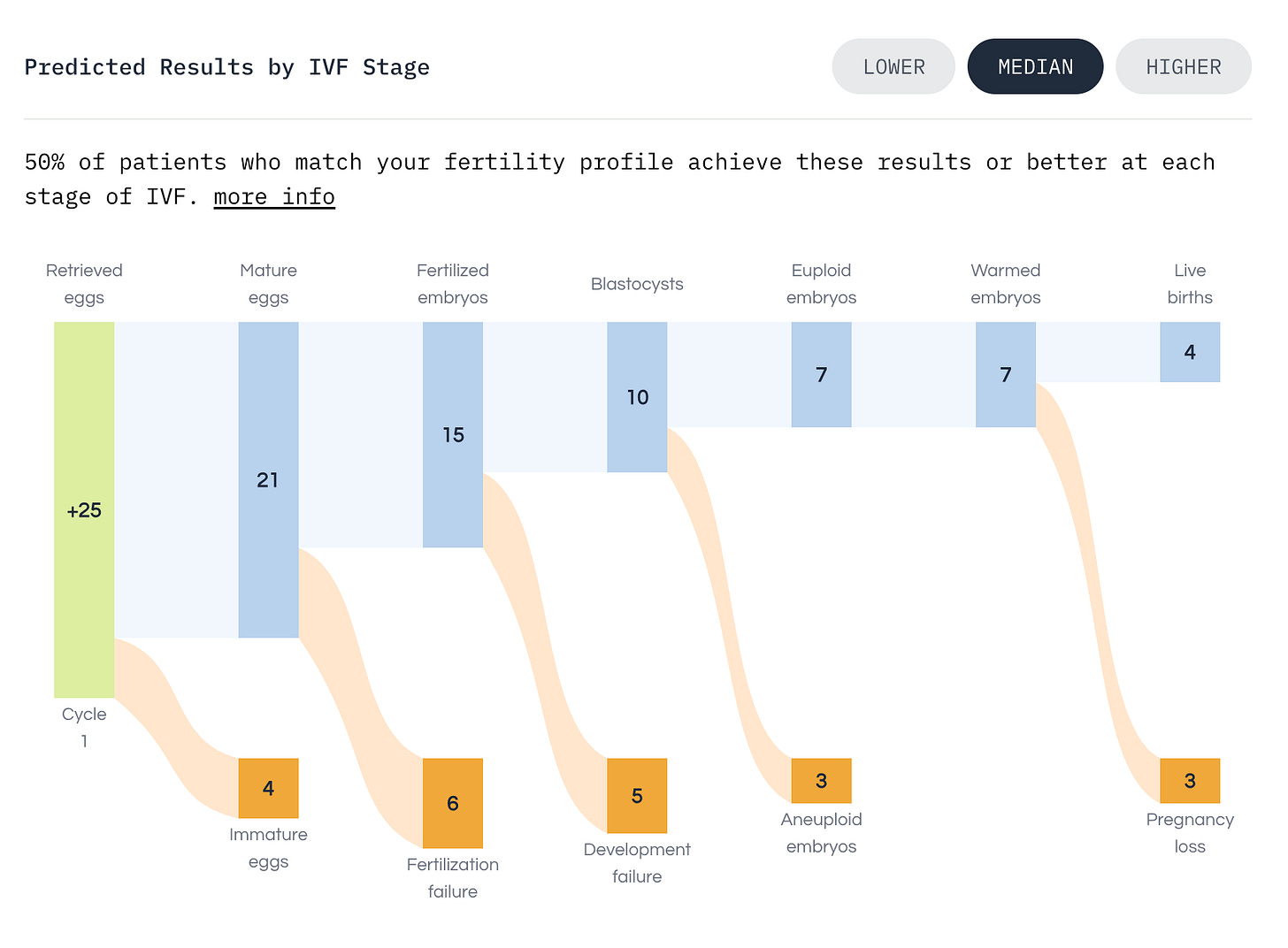
Consider a 35-year-old woman pursuing elective embryo freezing at a top-performing clinic. Before treatment, our model predicts a median of 4 and mean of 5 euploid embryos, with 80% of outcomes falling between 1 and 10. Her baseline probabilities are 88% for at least one live birth and 49% for five or more euploid embryos.
When retrieval results become available, all downstream predictions update:
With a low retrieval of 10 eggs, she can expect about 3 euploid embryos. Her probability of at least one live birth drops slightly to 85%, and her chance of getting five or more euploid embryos, often a target for families wanting genetic screening options, drops to just 14%.
With the expected retrieval of 17 eggs, she is on track for about 5 euploid embryos. Her probability of at least one live birth rises to 96%, and she has a 59% chance of getting five or more euploid embryos.
With a high retrieval of 25 eggs, she can expect about 7 euploid embryos. Her probability of at least one live birth reaches 99%, and she has a 90% chance of getting five or more euploid embryos, providing excellent options for family planning.
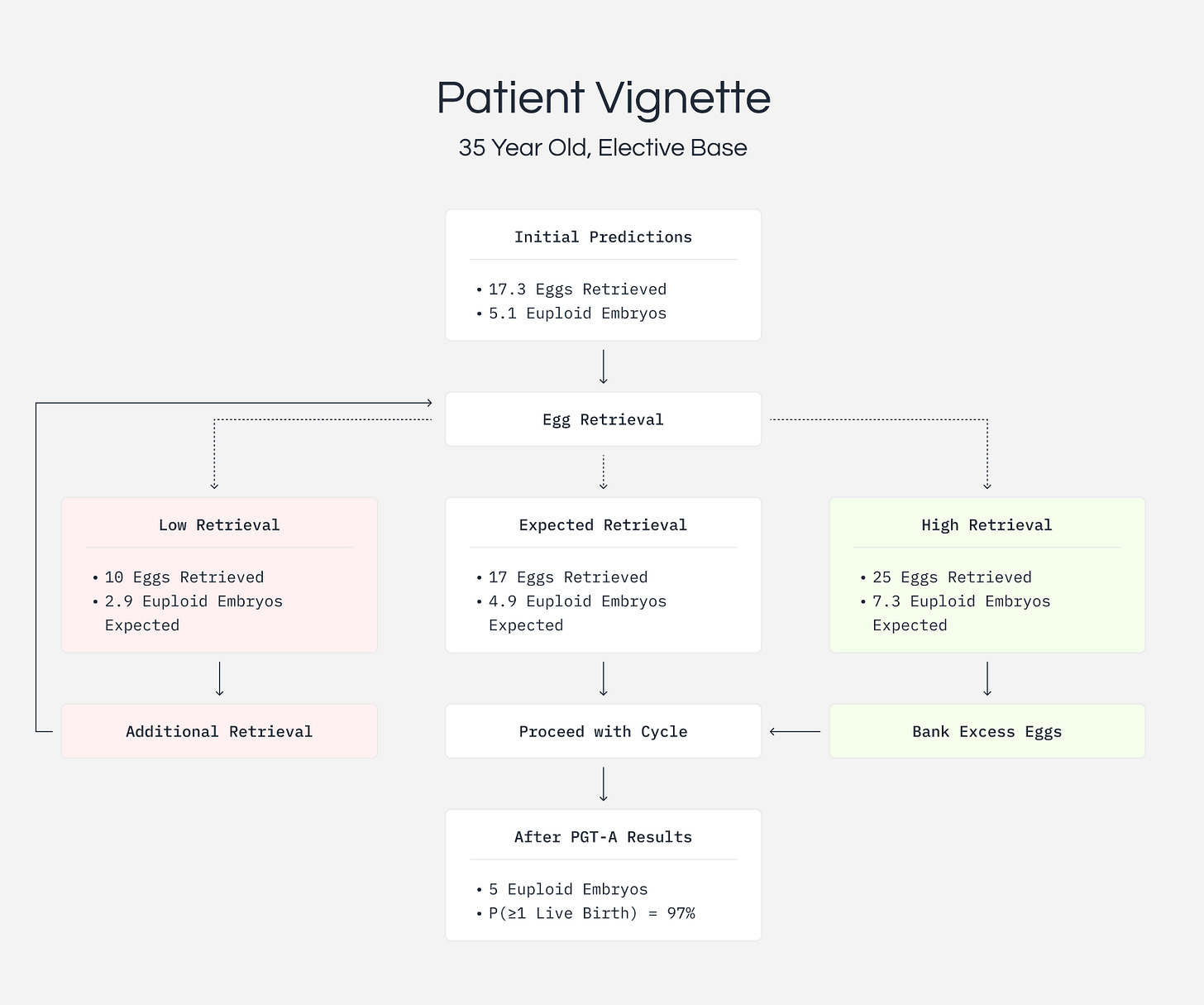
These shifts in probabilities inform clinical decisions. A drop from 49% to 14% for achieving five or more embryos provides quantitative justification for considering additional cycles. Conversely, a 90% probability suggests additional retrievals may be unnecessary.
Planning Multiple Cycles and Combining Frozen Eggs
Many patients today bank eggs across multiple ages or combine fresh cycles with previously frozen eggs. Our calculator handles this complexity while maintaining biological accuracy.
Consider a woman who froze 20 eggs at age 32 and now, at 35, is deciding whether to do another retrieval. The calculator shows her frozen eggs will likely yield about 6 euploid embryos, benefiting from her younger age’s 76% euploidy rate. A fresh cycle now would add about 5 more euploid embryos with her current age’s 71% euploidy rate. Combined, she can expect about 11 euploid embryos, enough for extensive family planning options.
Seven Fertility Profiles
Not all IVF patients are the same. A 35-year-old freezing eggs electively has very different odds than someone with endometriosis seeking treatment. We developed seven distinct fertility profiles to provide accurate predictions for a wide array of users:
Non-Elective Profiles
For traditional IVF patients with diagnosed infertility, we offer five profiles: ovulatory disorders (including PCOS), tubal factor, endometriosis, unexplained infertility, and male factor infertility. Each captures the unique facets of that diagnosis. For instance, PCOS patients often retrieve more eggs5, while male factor infertility primarily affects fertilization rates.
Elective Profiles
For elective patients pursuing fertility preservation or genetic testing without infertility, we created two profiles. The baseline profile gives conservative estimates for typical egg freezers, while the optimistic profile reflects the excellent outcomes often seen in egg donors who have been screened for high fertility potential.
Clinical Impact
Research indicates that patients often overestimate IVF success rates6. Our calculator enables more informed decision-making by providing accurate probability ranges. Understanding that 10 retrieved eggs yield a 14% chance of five or more euploid embryos, while 25 eggs increase this to 90%, allows patients and clinicians to evaluate the benefit of additional cycles objectively.
Limitations
While our model represents a significant advance, it has important limitations. We did not incorporate AMH (Anti-Müllerian Hormone) or AFC (Antral Follicle Count) as inputs, because the HFEA data we used for modeling egg retrieval does not track these values7. Additionally, our model assumes each patient has a single infertility diagnosis, though many couples face multiple contributing factors, as the HFEA data lacked enough patients with each combination of diagnoses to reliably model their interactions. The HFEA data itself imposed further constraints, reporting some outcomes in ranges like "6-10 eggs," requiring us to use the midpoint for calculations. Despite these limitations, our calculator still provides the most comprehensive and adaptive predictions currently available.
Conclusion
Our embryo calculator advances IVF prediction beyond static point estimates to dynamic, uncertainty-aware guidance. By maintaining full probability distributions and updating predictions with observed outcomes, patients can obtain personalized estimates that evolve throughout treatment. The calculator is freely available at herasight.com, putting these capabilities directly in the hands of patients and clinicians.
For researchers and clinicians interested in the technical details, the complete academic paper is available here.
Technical Appendix
To build our calculator, we analyzed data from over half a million IVF cases, combining real-world clinical registry data with insights from leading scientific studies. Specifically, we examined 103,924 IVF cycles reported by the UK's Human Fertilization and Embryology Authority (HFEA) from 2017-2018. This data allowed us to accurately model key early stages of IVF: how many eggs are retrieved, how many mature properly, and how many fertilize.
For later stages, which aren't detailed in the HFEA data, we integrated information from 435,291 observations published in reputable scientific journals. This included data on how many embryos successfully reach the blastocyst stage, rates of genetic normality (euploidy), survival after freezing and thawing (vitrification), and live birth rates. These studies provided reliable benchmarks, ensuring our predictions reflect real clinical outcomes.
Our model treats IVF as a step-by-step process, recognizing that at each stage, some eggs or embryos won't progress further. Initially, we predict how many eggs you might retrieve using statistical methods that account for the natural variability seen in real patient outcomes (so-called zero-inflated negative binomial regression). For subsequent stages, such as maturation, fertilization, embryo development, and genetic normality, we use established probability methods (binomial filters) to estimate how many eggs and embryos typically advance through each phase.
We also carefully consider age, recognizing its major impact on IVF success. For example, embryo quality often declines with age, and our calculator uses age-specific patterns drawn from clinical data to reflect this accurately. Rather than simply averaging outcomes, our approach generates a full range of possible results, providing realistic expectations for each stage of treatment.
We rigorously tested our calculator to ensure it performs reliably. In validation tests, our predictions closely matched actual patient outcomes, demonstrating minimal discrepancies between predicted and real-world results. Our probability ranges were also accurate: 95% of real-world outcomes fell within our predicted ranges. This means patients and clinicians can confidently rely on our estimates for informed decision-making throughout the IVF journey.
We describe our methodology in more detail in our whitepaper.
Simon Hanassab, Ali Abbara, Arthur C. Yeung, Margaritis Voliotis, Krasimira Tsaneva-Atanasova, Tom W. Kelsey, Geoffrey H. Trew, Scott M. Nelson, Thomas Heinis, and Waljit S. Dhillo. The prospect of artificial intelligence to personalize assisted reproductive technology. npj Digital Medicine, 7(1):55, March 2024. ISSN 2398-6352. doi: 10.1038/ s41746-024-01006-x. URL https://www.nature.com/articles/s41746-024-01006-x.Publisher: Nature Publishing Group.
S. Gameiro, J. Boivin, L. Peronace, and C.M. Verhaak. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Human Reproduction Update, 18(6):652–669, November 2012. ISSN 1355-4786. doi: 10.1093/humupd/dms031. URL https://doi.org/10.1093/humupd/dms031.
Celia Hoi Yan Chan, Bobo Hi Po Lau, Michelle Yi Jun Tam, and Ernest Hung Yu Ng. Preferred problem solving and decision-making role in fertility treatment among women following an unsuccessful in vitro fertilization cycle. BMC Women’s Health, 19(1):153, December 2019. ISSN 1472-6874. doi: 10.1186/s12905-019-0856-5. URL https://doi.org/10.1186/s12905-019-0856-5.
Marilyn M. Schapira, Kathlyn E. Fletcher, Mary Ann Gilligan, Elisabeth Hayes, Toni K. LaForest, Purushottam W. Laud, B. Alexendra Matthews, and Joan M. Neuner. A Framework for Health Numeracy: How Patients Use Quantitative Skills in Health Care. Journal of health communication, 13(5):501–517, 2008. ISSN 1081-0730. doi:10.1080/10810730802202169. URL https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4162627/.
Khaldoun Khamaiseh, Roba Bdeir, Mohammad Mahmoud Abukbeer, Rami Khamaiseh, Ali Nassar, Dina Nasser Al-Sawadha, Rasha Rakan Al Mraikhat, Marah Jamal Khraisat, Rana Radi Alawneh, and Omar Al-Mansour. Impact of Polycystic Ovary Syndrome Hyperandrogenic Phenotypes A and Non-Hyperandrogenic D on Pregnancy Outcomes After in vitro Fertilization (IVF)/Intracytoplasmic Sperm Injection (ICSI). International Journal of Women’s Health, 17:561–569, March 2025. ISSN 1179-1411. doi: 10.2147/IJWH.S500692. URL https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11884253/.
T Copp, D Kvesic, D Lieberman, D Bateson, and K J McCaffery. ‘Your hopes can run away with your realistic expectations’: a qualitative study of women and men’s decision-making when undergoing multiple cycles of IVF. Human Reproduction Open, 2020(4):hoaa059, December 2020. ISSN 2399-3529. doi: 10.1093/hropen/hoaa059. URL https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7757429/.
HFEA: UK fertility regulator. URL https://www.hfea.gov.uk/about-us/data-research/.